You can set up this STEM activity for yourself in just a few minutes – you only need a few simple ingredients! It’s quick and easy, and the wow factor will make you want to try the experiment over and over again. The bubbles and foam are SO COOL!
This post contains affiliate links. If you use these links to buy something we may earn a small commission which helps us run this website.
Helpful Tips:
What is elephant toothpaste?
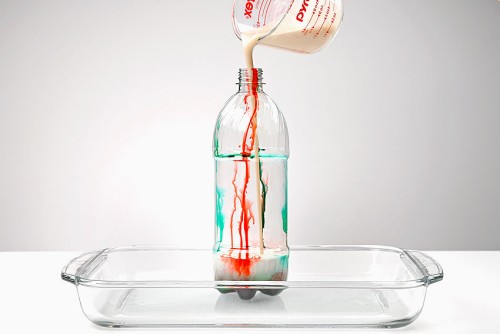
Elephant toothpaste is an easy science experiment for kids of all ages. The foaming chemical reaction looks just like toothpaste being squeezed out of a big tube! As the foam rises out of the plastic bottle, the stream of “toothpaste” is so big that it looks like it could be used by an elephant — hence the name!
How does elephant toothpaste work?
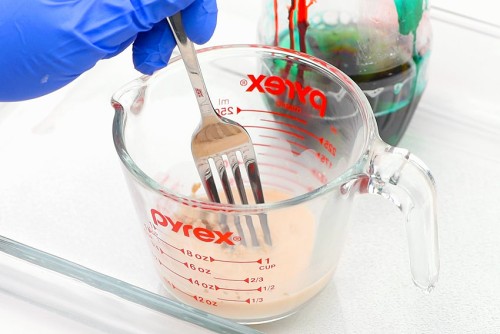
Elephant toothpaste is a chemical reaction that illustrates what happens when a catalyst causes the breakdown of hydrogen peroxide. Hydrogen peroxide is made up of both hydrogen and oxygen atoms, and with time it eventually breaks down into oxygen and water. Hydrogen peroxide is often found in dark brown bottles, which helps to slow down this process. When a catalyst is added, this usually slow reaction happens very quickly. Yeast contains an enzyme called “catalase”, which, when added to the hydrogen peroxide, rapidly releases the oxygen molecules. The dish soap helps increase the surface tension and causes the oxygen gas bubbles to get trapped and last longer — causing the foam reaction. This science experiment is a demonstration of rapid decomposition, and it also creates an exothermic reaction – or heat. If you put your hand close to the bottle you can feel the warmth created by the energy, and you might even see steam!
Can I use 3% hydrogen peroxide to make elephant toothpaste?
We definitely recommend using 12% hydrogen peroxide, or at the very least 6%, to get a good reaction between the ingredients. If you can only find 3% peroxide (the regular kind you can find in stores, for disinfecting cuts), your experiment will still work, it just won’t be as impressive. We tried it and it does create foam that comes out of the bottle, but the foam isn’t as thick and it moves slower – so it doesn’t shoot out from the bottle. Overall, it just doesn’t look as good. For the best (and coolest!) results you’ll want to use 12% hydrogen peroxide.
Where can I buy 12% hydrogen peroxide?
Look for 6% (also called 20 volume) liquid hydrogen peroxide, or 12% (40 volume). The higher the volume, the bigger and better the reaction will be.
Is it safe to touch elephant toothpaste?
Once the chemical reaction has occurred, it should be safe to touch the elephant toothpaste foam. When the hydrogen peroxide breaks down, all that remains is oxygen, water, and soap bubbles. However, any remaining hydrogen peroxide has hasn’t broken down may irritate your skin, so we still recommend only touching the foam with your gloves on.
Do I have to use food colouring in the elephant toothpaste recipe?
No, the food colouring along the sides of the bottle gives the experiment a fun toothpaste look, but you don’t have to add food colouring if you don’t want to! If you don’t like the look of the stripes, you could fully mix the food coloring into the peroxide and soap mixture. Instead of stripes in the foam, it will give you coloured foam.
Watch our video tutorial or follow our step by step written instructions to make this simple (and very cool!) experiment. Elephant toothpaste is such a fun experiment with a big foaming reaction! It’s great for school science lessons, at home, or even at STEM themed birthday parties.
Here’s even more science experiment ideas:
Dancing Popcorn Experiment
Bouncing Bubbles
Egg in Vinegar Experiment
Our book Low-Mess Crafts for Kids is loaded with 72 fun and simple craft ideas for kids! The projects are fun, easy and most importantly low-mess, so the clean up is simple!
Where to buy: